secure 100% marks
Chemistry is a scoring subject and students can obtain higher marks in this subject by putting in little hard work. Chemistry is a very important subject for both board exam and various competitive exams. One can score 95+ marks in this subject in board exam by investing little bit of time. Nowadays different competitive exams are conducted within one month after the completion of class 12 board exam. If you have command over this subject then you can really perform well in different competitive exams too.
Why students face difficulty in chemistry?
Many students fail to score good marks in this subject. The only reason behind it is that they fear from chemistry. If you really want to perform better in this subject then try to remove chemistry-phobia from your mind. Believe me, chemistry is one of the most easy subjects in the science stream provided you study this subject with full concentration and consistently. You must read chemistry regularly or you will fail to prepare it well for the board exam. Try to consult your teacher and friends when you find any topic difficult to understand.
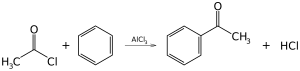
If you want to secure 95+ marks then you need to concentrate equally on all chapters of chemistry. Forweak students who want to get magical 23 marks i.e. passing marks or more, it’s important that they prepare selective but important topics. First of all thoroughly learn last three chapters of chemistryBiomolecules, Polymers and Chemistry in Everyday Life. These three chapters together carry 10 marks. Prepare all above mentioned topics of Organic chemistry
In Inorganic Chemistry, p-Block Elements and d-Block Elements together constitute 13 marks. Though it a tough ask to mug up both the chapters in a quick time, it would be ideal to study the notes of these chapters. The questions from the inorganic chemistry are directly asked. Students should practice the structures of different chemical compounds of p-block elements properly because they are often asked in the board exam. In summary, inorganic chemistry should be read in discrete manner and regularly. Because irregular study of inorganic chemistry can cost you very much and you tend to forget even the already learned topic too.
The last three chapters like Biomolecules, Polymer and Chemistry in Everyday life should be prepared really well. They together constitute 10 marks and students often dare to take these chapters lightly and it really affects their final marks in Chemistry.
Physical Chemistry should be read in appropriate manner. First of all, each formula from each chapter should be learned. Students should then try to solve related problems based on those formulas. Theory part should also be learned from those chapters as they are often asked in the exam.
Try to solve sample paper on every Sunday at least. It will help you in time management and would give you an idea of which question should be attempt first.
If you tend to forget the learned topics then do not take much stress. It happens with every student. Prepare a time table and take out at least 2 hours daily for revising the learned topic. A regular revision can help you in securing higher marks in Chemistry.
-