Friedel-Crafts alkylation
The Friedel-Crafts reactions, discovered by French alkaloid chemist Charles Friedel and his American partner, James Crafts, in 1877, is either the alkylation or acylation of aromatic compounds catalyzed by a Lewis acid. They are very useful in the lab for formation of carbon-carbon bonds between an aromatic nucleus and a side chain.
Source of electrophile
Friedel-Crafts alkylation is an example of electrophilic substitution in aromatic compounds. The electrophile is formed in the reaction of an alkyl halide with a Lewis acid. The Lewis acid polarizes the alkyl halide molecule, causing the hydrocarbon part of it to bear a positive charge and thus become more electrophilic.
CH3—Cl + AlCl3 → CH3+ + AlCl4−
or
CH3Cl + AlCl3 → CH3δ+Cl+Al−Cl3
(The carbon atom has a slight excess of positive charge, as the electronegative chlorine atom draws electron density towards itself. The chlorine atom has a positive charge, as it has formed a sub-ordinate bond with the aluminium atom. In effect, the Cl atom has lost an electron, while the Al atom has gained an electron. Therefore, the Al atom has a negative charge.)
Mechanism of alkylation
The polarized, electrophilic molecule then seeks to saturate its electron deficiency and forms a π-complex with the aromatic compound that is rich in π-electrons. Formation a π-complex does not lead to loss of aromaticity. The aromaticity is lost however in the σ-complex that is the next stage of reaction. The positive charge in the σ-complex is evenly distributed across the benzene ring.
C6H6 + CH3+ → C6H6+Br → C6H5Br + H+
The σ-complex C6H6+Br can be separated (it is stable at low temperatures), while the π-complex can not.
RestrictionS
- Deactivating functional groups, such as nitro (-NO2), usually prevent the reaction from occurring at any appreciable rate, so it is possible to use solvents such as nitrobenzene for Friedel-Crafts alkylation.
- Primary and secondary carbocations are much less stable than tertiary cations, so rearrangement typically occurs when one attempts to introduce primary and secondary alkyl groups onto the ring. Hence, Friedel-Crafts alkylation using n-butyl chloride generates the n-butylium cation, which rearranges to the t-butyl cation, which is far more stable, and the product is exclusively the t-butyl derivative. This may, in some cases, be circumvented through use of a weaker Lewis acid.
- The Friedel-Crafts reaction can not be used to alkylate compounds which are sensitive to acids, including many heterocycles.
- Another factor that restricts the use of Friedel-Crafts alkylation is polyalkylation. Since alkyl groups have an activating influence, substituted aromatic compounds alkylate more easily than the original compounds, so that the attempted methylation of benzene to give toluene often gives significant amounts of xylene and mesitylene. The usual workaround is to acylate first (see the following sections) and then reduce the carbonyl group to an alkyl group.
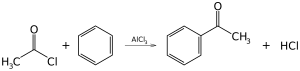
Friedel-Crafts acylation
Friedel-Crafts acylation, like Friedel-Crafts alkylation, is a classic example of electrophilic substitution.
Source of electrophile]Reacting with Lewis acids, anhydrides and chloranhydrides of acids become strongly polarized and often form acylium cations.
RCOCl + AlCl3 → RC+O + AlCl4-
Mechanism of acylation The mechanism of acylation is very similar to that of alkylation.
C6H6 + RC+O → C6H6—CO—R + H+
The ketone that is formed then forms a complex with aluminum chloride, reducing its catalytic activity.
C6H6—CO—R + AlCl3 → C6H6—C+(R)—O—Al−Cl3
Therefore, a much greater amount of catalyst is required for acylation than for alkylation.
QUESTIONS RELATED WITH TOPIC :
Which is most reactive in electrophilic substitution?
Question 3
Which gives a meta nitro compound as the main product upon nitration with a nitric acid-sulfuric acid mixture?
Question 6
Which gives a meta nitro compound as the main product upon nitration with a nitric acid-sulfuric acid mixture?
Question 8
Which is obtained as the main mononitration product upon reaction of m-t-butylanisole (1-t-butyl-3-methoxybenzene) with HNO3-H2SO4?
Question 10
Which of the following statements regarding electrophilic aromatic substitution is wrong?
Question 13
Which gives a para nitro compound as the main product upon nitration with a nitric acid-sulfuric acid mixture?
Question 14
Which gives a meta nitro compound as the main product upon nitration with a nitric acid-sulfuric acid mixture?
Question 15
Which of (a)-(d) does not give isopropylbenzene as a product upon reaction with benzene?
Question 16
Which combination of reagents used in the indicated order with benzene will give m-nitropropylbenzene?
Question 20
Which of the following statements regarding electrophilic aromatic substitution is wrong?

No comments:
Post a Comment